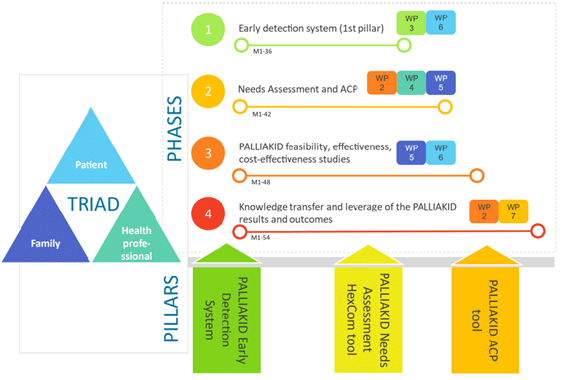
PALLIAKID methodological approach includes epistemologies, methods and techniques from different disciplines, and it is underpinned by the iterative participation of the patient-family caregiver-professional triad. Ethical issues and data protection are always relevant but are paramount in PPC. The project is structured in four research phases and eight WPs and lasts 54 months.
The main aim of this phase is to develop and clinically validate an Early Detection System (EDS) using machine learning (ML) models to identify children and adolescents in need of palliative care. The EDS will be developed in collaboration with healthcare professionals, data management roles, and ethics and data protection experts to ensure transparency, explainability, and fairness. The system will identify variables that correlate with the need for palliative care, apply exploratory data analytics and bio-computational engineering techniques, and develop predictive ML models using a retrospective dataset from the Hamburg University Hospital. The trained model will be tested in four clinical sites with non-anonymized prospective data to evaluate its feasibility and cost-effectiveness.
The main objective of this phase is to develop the PALLIAKID intervention and capacity-building program. The PALLIAKID intervention includes three components: the PALLIAKID Needs Assessment HexCom tool for comprehensive patient and family caregiver needs assessment, the PALLIAKID ACP tool for shared decision-making, and the PALLIAKID Patient Journey digital platform for patient empowerment and engagement. The platform will provide resources for needs assessment and advance care planning, document patient journeys, and enhance patient-centred communication with professionals. The PALLIAKID XR-based capacity-building program will train healthcare professionals on transversal skills and the use of intervention tools, aiming to improve their ability to support patients and family caregivers in palliative care.
The PALLIAKID intervention will be demonstrated in five clinical sites across Europe, selected based on geographical diversity, expertise in palliative care, and research capacity. A Randomized Clinical Trial (RCT) will be conducted involving 217 children and their families, with a primary outcome of changes in family caregivers’ well-being after 12 months of intervention. The trial will evaluate the effectiveness, cost-effectiveness, and feasibility of the PALLIAKID intervention, which includes the Needs Assessment HexCom tool, ACP tool, and Patient Journey digital platform. The PALLIAKID XR-based capacity-building program will also be evaluated for its effectiveness. The study will recruit a total of 310 families, with participants randomised into a control group (standard-of-care) or intervention group (PALLIAKID intervention) with an allocation ratio of 1:1. Participants will be followed for 12 months, during which they will use the PALLIAKID tools.
PALLIAKID aims to engage patients, family caregivers, and professionals throughout the project through a Patient, Families and Stakeholder Engagement Plan (PPEP) that follows equity, diversity, and inclusion principles. The plan includes co-creation activities, public engagement actions, and the establishment of two independent boards: the Young People Board (YPB) and the Families Advisory Board (FAB). The boards will provide feedback and input on project activities, and their experiences will be recorded following NICE guidelines. A Public Engagement Strategy will also be implemented to raise awareness of palliative care, share project results with the general public, and involve children, adolescents, and families in need of palliative care. The project will develop policy recommendations, strategies, and guidelines for patient-centred communication and standards based on knowledge generated throughout the project.