Work packages
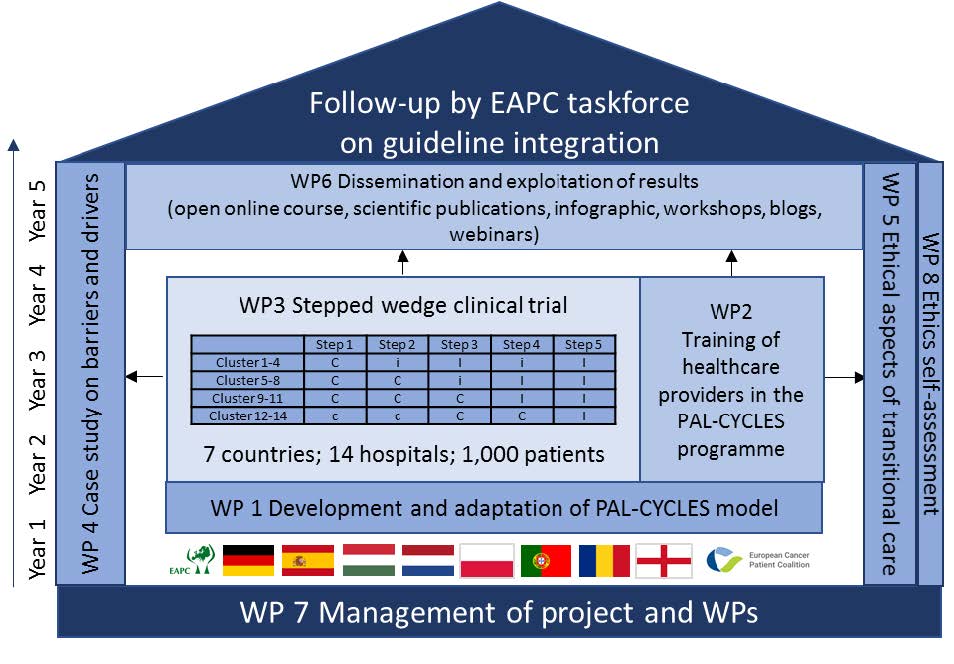
WP 1 - Development and local adaptation of the Pal-Cycles framework intervention
ULANC leads this WP which aims:
1. To perform an updated literature review on palliative care integration into cancer guidelines and current recommendations and evidence for transitional palliative care.
2. To co-ordinate the co-design of the standardized Pal-Cycles intervention for all partner countries including agreement on core components.
3. Undertake cross-cultural adaptation and oversee translation of the intervention to identify key elements for future training, implementation and testing.
WP 2 - Training and implementation support
HCS leads this WP which aims:
1. To develop an open access blended training program on hospital to community transitional care for advanced cancer patients and families.
2. To increase capacity at national level to deliver the education program on transitional palliative care programs, by organizing a train the trainer program for 7 partner countries implementing Pal- Cycles interventions.
3. To implement the education programs for minimum 140 healthcare professionals from the 7 partner countries implementing the clinical step wedged clinical trial.
4. To develop an online toolbox of materials that are supportive for the implementation of the transitional hospital community palliative care program for advanced cancer patients.
5. To assess the impact of the Pal-Cycles training program on the healthcare professionals.
WP 3 - Stepped wedge clinical trial
Radboudumc leads this WP which aims:
1.To perform a stepped wedge clinical trial in seven countries. A stepped wedge trial is a cluster-based trial where all sites eventually will receive the intervention. This will be implemented in all seven countries, in two hospital sites per country to provide a large-scale demonstration for transitional palliative care.
2.To undertake the analysis of effectiveness and cost effectiveness of the trial. Based on the data as collected in CASTOR two analysis will be prepared. The first concerns the primary and secondary effectiveness outcomes, and the second concerns the cost-effectiveness analysis.
3.To publish the results of the trial. After analysis of the data, the results will be described in a report highlighting the results of the (cost) effectiveness of the Pal-Cycles study. The report will be further disseminated via a scientific article.
WP 4 - Barriers and facilitators for planning and developing the Pal-Cycles intervention
UNAV leads this WP which aims:
1.To identify grey literature investigation like national or regional policy documents (strategies, plans, guidelines) on transitional care from oncology to palliative care approach in the several countries.
2.To explore the barriers/challenges and facilitators during the planification of the Pal-Cycles intervention adapted towards local situations and circumstances to optimize.
3.To understand different health professionals’ experiences on the Pal-Cycles local intervention.
WP 5 - Ethical, sociocultural and policy analysis
UCP leads this WP which aims:
1. To provide the conceptual and practical research ethics frameworks to ensure that Pal-Cycles is performed
according to responsible conduct of research and guarantee that research within this project is performed with the highest ethical standards.
2. To explore the ethical and sociocultural challenges surrounding transitional palliative care and the implementation of the Pal-Cycles programme.
3. To describe and explain how patients, their families and healthcare professionals experience the (gradual) transition from active cancer treatments towards palliative care and how they value the Pal-Cycles framework using an ethics of care perspective.
4. To describe and understand how stakeholders experience, value and perceive the ethical principle of justice (distributive and procedural) and equity (horizontal and vertical) in the access and allocation (e.g., resources and decisions) to transitional palliative care across European countries, taking into account sociocultural features, health systems and policies.
WP 6 - Dissemination and exploitation
EAPC leads this WP which aims:
1. To disseminate updates and findings to the relevant medical and healthcare practitioners.
2. To include and raise awareness among people affected by cancer and their families in the ensuring broad dissemination of results.
3. To deliver freely accessible information on how to better support patients to clinicians, patients, parents, family caregivers, the public and policy and decision makers.
4. To provide an ongoing knowledge exchange between other relevant EU project groups conducting research in
palliative and cancer care and related areas.
5. To influence policy and decision makers and funders of care.
6. To support all partners in the internal and external dissemination and communication aspects of the study, and maintain
a record of all activities.



